Respira, the new ventilator device developed gets the go ahead from the AEMPS for clinical trials
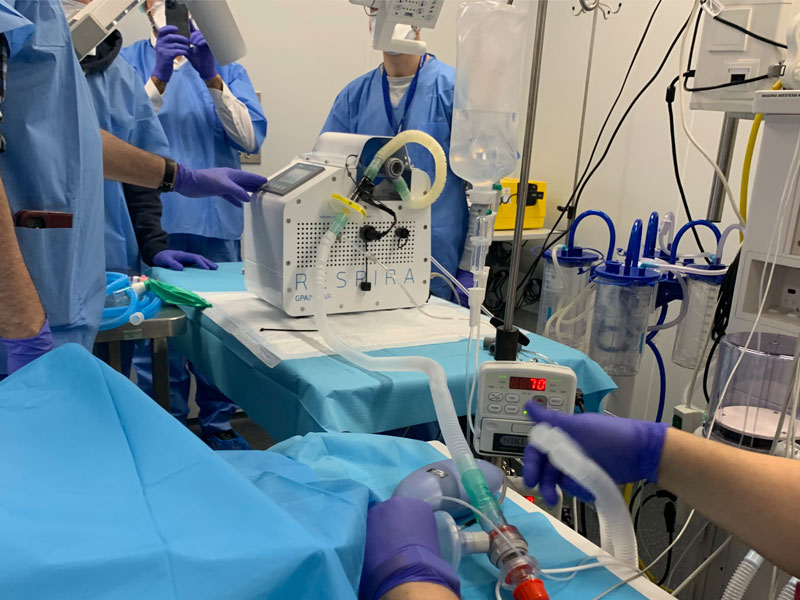
- The Spanish Agency of Medicines and Medical Devices (AEMPS) has approved a clinical trial with the ventilation device RESPIRA developed by GAPInnova with the support of professionals from the Hospital Clínic Barcelona, the Germans Trias I Pujol Research Institute and the University of Barcelona
- This is the second device developed with the support of these organizations to be approved by the AEMPS for testing on patients at the Hospital Clinic Barcelona and at Can Ruti
- The prototype of the RESPIRA device mechanizes manual ressusitations with AMBU devices providing automatic respiration assistance and monitoring
- The support received from the public, companies and administrations has been the key to the development of the design and industrical scaling up of this model, which will contribute to the provision of the Health System with the ventilation devices it needs
The Spanish Agency of Medicines and Medical Devices (AEMPS) has authorized a clinical trialwith the emergency ventilation device RESPIRA designed by GAPInnova. This is the second test developed with the support and experience of the Hospital Clinic Barcelona, the Germans Trias i Pujol Research Institute (IGTP) and the Faculty of Medicine and Health Sciences of the Universithy of Barcelona (UB) to be approved.
The clinical trial will allow the device to be tested in patients. It will take place initially at the Hospital Clínic and Can Ruti and will be extended to other hospitals in Catalalonia and the rest of Spain when data from the first patients is in.
This initiative has been made possible thanks to the support received from a large number of private individuals, companies and entities from civil society. It has also received the support of the Catalan Health Service which has brought about the inclusion of companies and has provided support to the centres looking to provide these solutions.
A new ventilator device to fight COVID-19
The RESPIRA device automates manual reanimation devices (BVM or AMBU), currently found in most health centres, and provides mechanical assistance to the patient. It has the functions needed for real-time monitoring of variables for individual patients by remote control to allow for its easy management in hospitals.
The device contains the electronics needed to be able to manage remote control of variables such as frequency and volume of air and the amount of oxygen provided to the patient. These are provided by SIEMENS and a precision drive unit manufactured by SMC.
On Tuesday 31 March the validation trials with a simulator were completed at the Hospital Clínic Barcelona and on 1 April tests on animals took place at the Centre for Comparative Medicine and Biomage (CMCiB) of the IGTP at Can Ruti. In parallel, as required by the AEMPS instructions, electromagnetic compatibility tests have been carried out to ensure that the device does not cause interference for other equipment in hospitals. Today it has received the authorization for a clinical trial with patients with COVID-19.
The team supervising the simulations consists of Dr. Josep M. Nicolás, specialist in intensive care at the Hospital Clinic Barcelona and professor at the UB and Dr Ramón Farré, Professor of Physiology at the UB and leader of the group in Respiratory Biophysics and Engineering at the IDIBAPs and Dr Manel Puig of the Germans Trias i Pujol Research Institute (IGTP).
Other members of the team include Joan Grasas, health technology entrepreneur and innovatin consultant affiliated with the Catalan Health Institute (ICS) and Dr Jaume Pérez Payarols, Dr Martí Pons, Dr Maria Cols and Dr Arnau Valls from the Sant Joan de Déu Hospital in Barcelona.
At the moment the production team is preparing for manufactura as soon as the AEMPS confirms the certification of the device. GPAInnova calculates that initially more than 100 units can be produced daily and between 700 and 1,000 per week when production is completely industrialized. The Catalan company MAM is collaborating in the industrialization process being carried out in the factory in Santa Perpetua de Mogoda.
