The CMCiB awarded the certificate for Good Laboratory Practice (GLP) for preclinical research
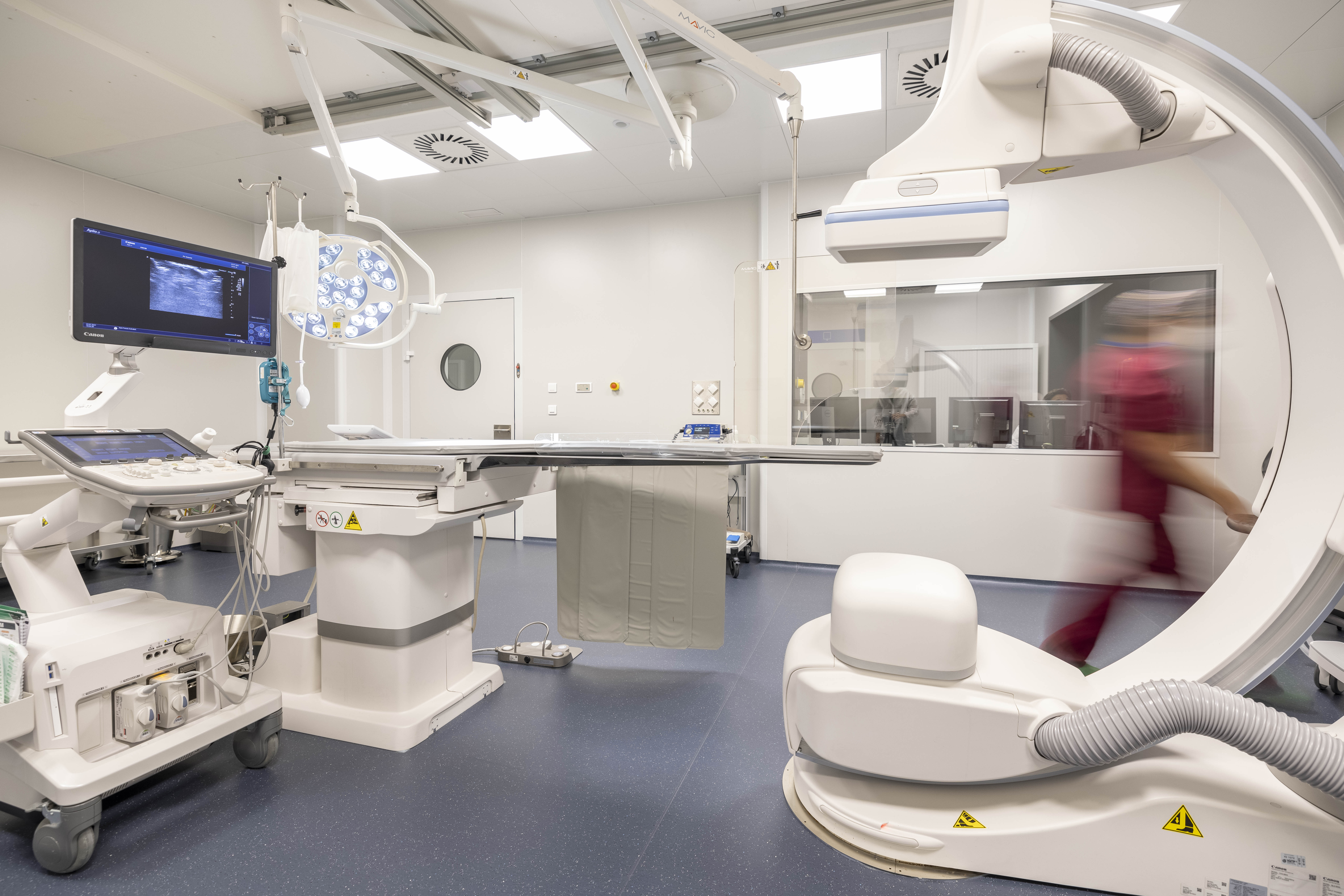
The Centre for Comparative Medicine and Bioimage (CMCiB) of the Germans Trias I Pujol Research Institute has been awarded the certificate for complying with good laboratory practices (GLP) in line with the European Directive 2004/9/CE. The centre complies with the principles established by the Spanish Royal Decree 822/19932 for carrying out preclinical studies with healthcare products (analytical tests, clinical chemistry - haematology and biochemistry - in studies administrating trial products, obtaining samples and biocompatibility of healthcare products). The areas certified are the surgical and bioimage areas, including the technology of vascular radiology interventions, ultrasound scan and magnetic resonance.
This certificate, awarded by the Government of Catalonia, guarantees the quality and integrity of the results of the research laboratories that carry out preclinical studies. The regulatory milestone demonstrates that the CMCiB adheres to international research standards. Since 2021, the legislation requires that medical devices, such as prosthetics, catheters, valves, stents and surgeries etc. are preclinically validated under the GLP certification before initiating clinical trials.
To obtain this certification, the CMCiB has included three more quality control professionals on its staff and the Quality Guarantee Unit of FalcoQuality has provided support. This took the form of training for personnel on the principles of GLP, advice on the implantation process and the execution of all the actions in the quality guarantee programme. The team of professionals at the CMCiB has been working for the last year and a half to implant the quality system. The certificate obtained helps to situate the CMCiB-IGTP as a reference centre for carrying out preclinical trials for medical devices in the conditions required by the regulatory agencies, helping companies with the process of bringing this type of product to market.
Good Laboratory Practice (GLP)
The principles of good laboratory practice (GLP) outline a system of quality for the organization and conditions in which research laboratories plan, carry out, monitor, register and store substances and chemical products. These studies are necessary to be able to register and/or authorize chemical products for use as medication, both for human and veterinary use, cosmetics, healthcare products, pesticides and phytosanitary products, human and animal food additives and industrial chemical substances.
Since 1995 the organization that oversees compliance with the GLP principles for studies on healthcare and environmental safety of medicines and healthcare products for the Organisation for Economic Co-operation and Development (OECD) and the European Union is the Ministry of Health of the Government of Catalonia. Specifically, the Pharmaeutical and Healthcare Products Control Service of the Directorate General of Healthcare Regulation.
The CMCiB is a technological centre that supports biomedical research mainly in the areas of surgery, diagnostic imaging, infectious diseases and cancer. It uses animal models when necessary, always promoting responsible research through the 3Rs Programme, which includes the use of zebrafish, Drosophila, mathematical modelling and bioimaging and animal welfare programmes.
